QuantiChrom™ Iron Assay Kit
Application
- For quantitative determination of iron ions Fe3+ and/or Fe2+ and evaluation of drug effects on iron metabolism.
Key Features
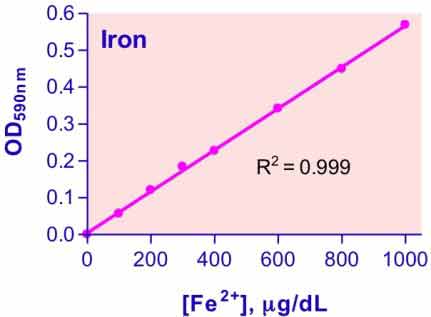
- Sensitive and accurate. Linear detection range 27 µg/dL (4.8 µM) to 1,000 µg/dL (179 µM) iron in a 96-well plate assay.
- Simple and high-throughput. The procedure involves the addition of a single working reagent and incubation for 40 min. Can be readily automated as a high-throughput assay for thousands of samples per day.
- Improved reagent stability and versatility. The optimized formulation has greatly enhanced the reagent and signal stability. Cuvette or 96-well plate assay.
- Low interference in biological samples. No pretreatments are needed. Assays can be directly performed on serum samples.
Method
- OD590nm
Samples
- Biological (e.g. serum) and environmental samples
Species
- All
Procedure
- 30 min
Size
- 250 tests
Detection Limit
- 27 µg/dL (4.8 µM)
Shelf Life
- 12 months
More Details
Iron level in blood is a reliable diagnostic indicator of various disease states. Increased levels of iron concentration in blood are associated with blood loss, increased destruction of red blood cells (e.g. hemorrhage) or decreased blood cell survival, acute hepatitis, certain sideroachrestic anemias, ingestion of iron-rich diets, defects in iron storage (e.g. pernicious anemia). Decreased levels of blood iron may result from insufficient iron ingestion from diets, chronic blood loss pathologies, or increased demand for iron storage during normal pregnancy. Simple, direct, and automation-ready procedures for measuring iron concentrations find wide applications in research, drug discovery, and environmental monitoring. BioAssay Systems iron assay kit is designed to measure total iron directly in serum without any pretreatment. The improved method utilizes a chromogen that forms a blue-colored complex specifically with Fe2+. Fe3+ in the sample is reduced to Fe2+, thus allowing the assay for total iron concentration. The intensity of the color, measured at 590nm, is directly proportional to the iron concentration in the sample.I would like to know how serum samples should be prepared for use in the assay.
Sera are usually obtained by centrifugation of clotted blood (fresh blood standing for about 30 min at room temperature). Both, fresh serum or frozen serum can be assayed directly (50 µL sample + 200 µL working reagent).
Do DTT or IGEPAL/NP-40 in cell lysis buffer will have any influence on the assay?
Our data show that up to 4% NP-40 has no effect on the DIFE-250 assay. Up to 20 mM β-mercaptoethanol also had no effect on the DIFE-250. We did not test DTT, but we believe DTT would not interfere with this assay either.
Can you use DIFE-250 to measure iron in whole blood?
No, whole blood needs to be diluted to a degree that the normal serum iron concentration is below the detection limit of the assay. The assay is not suitable for measuring hemoglobin bound iron.
Please let us know the preparation for the liver tissue.
Tissue samples should be dried overnight at 106°C and weighed. Samples can then be solubilized in 6N nitric acid by heating at 100°C to release protein-associated iron. The solution is neutralized with NaOH, diluted in deionized water as necessary, and assayed for iron concentration using the DIFE-250 kits.
Hendricks, MR et al (2021). Extracellular vesicles promote transkingdom nutrient transfer during viral-bacterial co-infection. Cell Reports, 34(4), 108672. Assay: Iron in human extracelllular vesicles.
Hassan AT, Kwong R (2020). The neurophysiological effects of iron in early life stages of zebrafish. Environmental Pollution, 267, 115625. Assay: Iron in water.
Liao, D et al (2020). Identification of pannexin 2 as a novel marker correlating with ferroptosis and malignant phenotypes of prostate cancer cells. OncoTargets and Therapy, 13, 4411-4421. Assay: Iron in human prostate cancer cell lysates.
Salama SA, Omar HA (2021). Modulating NF-kB, MAPK, and PI3K/AKT signaling by ergothioneine attenuates iron overload-induced hepatocellular injury in rats. Journal of Biochemical and Molecular Toxicology, e22729. Assay: Iron in rat.
Elhassanny, AEM et al (2020). Heme-dependent er stress apoptosis: A mechanism for the selective toxicity of the dihydroartemisinin, nsc735847, in colorectal cancer cells. Frontiers in Oncology, 10. Assay: Iron in human colon cancer cell lysates.
Shan, Y et al (2020). Ubiquitin-like modifier activating enzyme 1 as a novel diagnostic and prognostic indicator that correlates with ferroptosis and the malignant phenotypes of liver cancer cells. Frontiers in Oncology, 10, 592413. Assay: Iron in human liver cancer cell lysates.
Chen, K et al (2019). Transcription analysis of the stress and immune response genes to temperature stress in ostrinia furnacalis. Frontiers in Physiology, 10, 1289. Assay: Iron in ostrinia furnacalis plasma.
Hernandez, EP et al. (2020). Expression analysis of glutathione S-transferases and ferritins during the embryogenesis of the tick Haemaphysalis longicornis. Heliyon, 6(3), e03644. Assay: Iron in haemaphysalis longicornis egg homogenates.
Deng, Q et al (2021). Salmonella effector SpvB aggravates dysregulation of systemic iron metabolism via modulating the hepcidin-ferroportin axis. Gut Microbes, 13(1), 1-18. Assay: Iron in mouse serum and liver.
Tang, LJ et al (2021). Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion. Free Radical Biology & Medicine, 162, 339-352. Assay: Iron in rat cardiac tissue homogenates.
Sanyear, C et al (2020). Iron homeostasis in a mouse model of thalassemia intermedia is altered between adolescence and adulthood. PeerJ, 8, e8802. Assay: Iron in mouse serum.
Lyu, S et al (2020). Deficiency of Meis1, a transcriptional regulator, in mice and worms: Neurochemical and behavioral characterizations with implications in the restless legs syndrome. Journal of Neurochemistry, 155(5), 522-537. Assay: Iron in mouse serum.
Furihata, T et al (2021). Cardiac-specific loss of mitoNEET expression is linked with age-related heart failure. Communications Biology, 4(1), 138. Assay: Iron in mouse mitochondria.
Lyu, S et al (2020). Mu opioid receptor knockout mouse: Phenotypes with implications on restless legs syndrome. Journal of Neuroscience Research, 98(8), 1532-1548. Assay: Iron in mouse.
Matte JJ, Audet I (2020) Maternal perinatal transfer of vitamins and trace elements to piglets. Animal, 14(1), 31-38. Assay: Iron in pig serum.
Yang, B et al (2020). Ferrous-iron-activated transcriptional factor adhr regulates redox homeostasis in clostridium beijerinckii. Applied and Environmental Microbiology, 86(7). Assay: Iron in clostridium beijerinckii AdHr protein.
Pease, NA et al (2020). Dek expression in breast cancer cells leads to the alternative activation of tumor associated macrophages. Cancers, 12(7). Assay: Iron in mouse cell lysates.
Oliveira, R et al (2020). The novel ECF56 SigG1-RsfG system modulates morphological differentiation and metal-ion homeostasis in Streptomyces tsukubaensis. Scientific Reports, 10(1), 21728. Assay: Iron in streptomyces tsukubaensis cell culture supernatant.
Santiago Gonzalez, DA et al (2019). Iron metabolism in the peripheral nervous system: The role of dmt1, ferritin, and transferrin receptor in schwann cell maturation and myelination. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 39(50), 9940-9953. Assay: Iron in mouse DRG explant cultures and sciatic nerve homogenates.
Dichtl, S., Demetz, E., Haschka, D., Tymoszuk, P., Petzer, V., Nairz, M. & Theurl, I. (2019). Dopamine Is a Siderophore-Like Iron Chelator That Promotes Salmonella enterica Serovar Typhimurium Virulence in Mice. mBio, 10(1), e02624-18. Assay: Iron in mice serum.
Malhotra, H., Kumar, M., Chauhan, A. S., Dhiman, A., Chaudhary, S., Patidar, A. & Raje, M. (2019). Moonlighting Protein Glyceraldehyde-3-Phosphate Dehydrogenase: A Cellular Rapid-Response Molecule for Maintenance of Iron Homeostasis in Hypoxia. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology, 52(3), 517-531. Assay: Iron in mouse cells.
Cheli, V. T., Gonzalez, D. A. S., Marziali, L. N., Zamora, N. N., Guitart, M. E., Spreuer, V. & Paez, P. M. (2018). The Divalent Metal Transporter 1 (DMT1) is required for iron uptake and normal development of oligodendrocyte progenitor cells. Journal of Neuroscience, 38(43), 9142-9159. Assay: Iron in mice cells.
Samba Mondonga, M., Calve, A., Mallette, F. A., & Santos, M. M. (2018). MyD88 regulates the expression of SMAD4 and the iron regulatory hormone hepcidin in hepatoma cells. Frontiers in cell and developmental biology, 6, 105. Assay: Iron in human kidney cells.
Worley, B. L., Kim, Y. S., Mardini, J., Zaman, R., Leon, K. E., Vallur, P. G. & Phaeton, R. (2018). GPx3 supports ovarian cancer progression by manipulating the extracellular redox environment. Redox Biology pii: S2213-2317(18)30891-7. Assay: Iron in human ovarian cells.
Asshoff, M., Petzer, V., Warr, M. R., Haschka, D., Tymoszuk, P., Demetz, E. & Fowles, P. (2017). Momelotinib inhibits ACVR1/ALK2, decreases hepcidin production, and ameliorates anemia of chronic disease in rodents. Blood, 129(13), 1823-1830. Assay: Iron in rat/mice serum.
Ma, X., Pham, V. T., Mori, H., MacDougald, O. A., Shah, Y. M., & Bodary, P. F. (2017). Iron elevation and adipose tissue remodeling in the epididymal depot of a mouse model of polygenic obesity. PloS one, 12(6), e0179889. Assay: Iron in mice serum.
Marks, E. S., Bonnemaison, M. L., Brusnahan, S. K., Zhang, W., Fan, W., Garrison, J. C., & Boesen, E. I. (2017). Renal iron accumulation occurs in lupus nephritis and iron chelation delays the onset of albuminuria. Scientific reports, 7(1), 12821. Assay: Iron in mice plasma.
Quan, Y. Y., Liu, Y. H., Lin, C. M., Wang, X. P., & Chen, T. S. (2017). Peroxynitrite dominates sodium nitroprusside-induced apoptosis in human hepatocellular carcinoma cells. Oncotarget 8(18): 29833-29845. Assay: Iron in cells.
Shang, Y. M., Wang, G. S., Sliney, D. H., Yang, C. H., & Lee, L. L. (2017). Light-emitting-diode induced retinal damage and its wavelength dependency in vivo. International journal of ophthalmology 10(2): 191-202. Assay: Iron in Sprague Dewley rats retina protein.
Sun, X., Zhao, Y., Jia, J., Xie, J., Cheng, J., Liu, H. & Fu, Y. (2017). Uninterrupted expression of CmSIT1 in a sclerotial parasite Coniothyrium minitans leads to reduced growth and enhanced antifungal ability. Frontiers in microbiology, 8, 2208. Assay: Iron in Coniothyrium minitans cells.
Zhang, C. W., Tai, Y. K., Chai, B. H., Chew, K. C., Ang, E. T., Tsang, F. & Lim, K. L. (2017). Transgenic mice overexpressing the divalent metal transporter 1 exhibit iron accumulation and enhanced parkin expression in the brain. Neuromolecular medicine, 19(2-3), 375-386. Assay: Iron in mouse feces.
Hendricks, M. R., Lashua, L. P., Fischer, D. K., Flitter, B. A., Eichinger, K. M., Durbin, J. E. & Bomberger, J. M. (2016). Respiratory syncytial virus infection enhances Pseudomonas aeruginosa biofilm growth through dysregulation of nutritional immunity. Proceedings of the National Academy of Sciences, 113(6), 1642-1647. Assay: Iron in human cells.
Karoopongse, E., Marcondes, A. M., Yeung, C., Holman, Z., Kowdley, K. V., Campbell, J. S., & Deeg, H. J. (2016). Disruption of iron regulation after radiation and donor cell infusion. Biology of Blood and Marrow Transplantation, 22(7), 1173-1181. Assay: Iron in mouse serum.
Li, Y., Pan, K., Chen, L., Ning, J. L., Li, X., Yang, T. & Tao, G. (2016). Deferoxamine regulates neuroinflammation and iron homeostasis in a mouse model of postoperative cognitive dysfunction. Journal of neuroinflammation, 13(1), 268. Assay: Iron in mice cells.
Noguchi-Sasaki, M., Sasaki, Y., Shimonaka, Y., Mori, K., & Fujimoto-Ouchi, K. (2016). Treatment with anti-IL-6 receptor antibody prevented increase in serum hepcidin levels and improved anemia in mice inoculated with IL-6-producing lung carcinoma cells. BMC Cancer 16: 270. Assay: Iron in mouse serum.
Tamayo, E., Benabdellah, K., & Ferrol, N. (2016). Characterization of three new glutaredoxin genes in the arbuscular mycorrhizal fungus Rhizophagus irregularis: putative role of RiGRX4 and RiGRX5 in iron homeostasis. PloS one, 11(2), e0149606. Assay: Iron in yeast cells.
Theurl, I., Hilgendorf, I., Nairz, M., Tymoszuk, P., Haschka, D., Asshoff, M. & Sopper, S. (2016). On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nature medicine, 22(8), 945. Assay: Iron in human plasma.
Anderson ER, et al (2011). Intestinal hypoxia-inducible factor-2alpha (HIF-2alpha) is critical for efficient erythropoiesis. J Biol Chem. 286(22):19533-40. Assay: Iron in mice serum.
Hamlin F, Latunde-Dada GO (2011). Iron bioavailibity from a tropical leafy vegetable in anaemic mice. Nutr Metab (Lond). 8:9. Assay: Iron in mice serum.
Ringseis R, et al (2010). Low availability of carnitine precursors as a possible reason for the diminished plasma carnitine concentrations in pregnant women. BMC Pregnancy Childbirth.10:17. Assay: Iron in human plasma.
Zhang Y, et al (2010). Diphthamide biosynthesis requires an organic radical generated by an iron-sulphur enzyme. Nature 465(7300):891-6. Assay: Iron in bacterial cell .
Zhu W, et al (2010). Genetic iron chelation protects against proteasome inhibition-induced dopamine neuron degeneration. Neurobiol Dis. 37(2):307-13. Assay: Iron in human neuron cell.
Chen H, et al (2009). Changes in iron-regulatory proteins in the aged rodent neural retina. Neurobiol Aging. 30(11):1865-76. Assay: Iron in rat serum.
Chen H, et al (2009). Dysfunction of the retinal pigment epithelium with age: increased iron decreases phagocytosis and lysosomal activity. Invest Ophthalmol Vis Sci. 50(4):1895-902. Assay: Iron in rat serum.
Shah, YM et al (2009). Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 9(2):152-64. Assay: Iron in mouse erythrocytes.
Sudarshan S, et al (2009). Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and hypoxia-inducible transcription factor 1alpha stabilization by glucose-dependent generation of reactive oxygen species. Mol Cell Biol. 29(15):4080-90. Assay: Iron in human tumor cell.
Yokosho K, et al (2009). OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiol. 149(1):297-305. Assay: Iron in plant rice.
Bandyopadhyay S, et al (2008). A proposed role for the Azotobacter vinelandii NfuA protein as an intermediate iron-sulfur cluster carrier. J Biol Chem. 283(20):14092-9. Assay: Iron in bacterial cell protein.
Raulfs EC, et al (2008). In vivo iron-sulfur cluster formation. Proc Natl Acad Sci U S A. 105(25):8591-6. Assay: Iron in bacterial cell protein.
Habel ME, Jung D. (2006) c-Myc over-expression in Ramos Burkitt’s lymphoma cell line predisposes to iron homeostasis disruption in vitro. Biochem Biophys Res Commun. 341(4):1309-16. Assay: Iron in human lymphoma cell.
To find more recent publications, please click here.
If you or your labs do not have the equipment or scientists necessary to run this assay, BioAssay Systems can perform the service for you.
– Fast turnaround
– Quality data
– Low cost
Please email or call 1-510-782-9988 x 2 to discuss your projects.
$479.00
For bulk quote or custom reagents, please email or call 1-510-782-9988 x 1.
Orders are shipped the same day if placed by 2pm PST
Shipping: RT
Carrier: Fedex
Delivery: 1-2 days (US), 3-6 days (Intl)
Storage: 4°C upon receipt
Related Products
You may also like…
| Name | SKU | Price | Buy |
|---|---|---|---|
| QuantiChrom™ Calcium Assay Kit | DICA-500 |
$439.00 |
|
| QuantiChrom™ Copper Assay Kit | DICU-250 |
$539.00 |
|
| QuantiChrom™ Magnesium Assay Kit | DIMG-250 |
$469.00 |
|
| QuantiChrom™ Chloride Assay Kit | DICL-250 |
$389.00 |
|
| QuantiChrom™ Chromium Assay Kit | DCRM-250 |
$509.00 |
Why BioAssay Systems
Quality and User-friendly • Expert Technical Support • Competitive Prices • Expansive Catalogue • Trusted Globally
