QuantiChrom™ Peroxide Assay Kit
Application
- For quantitative determination of peroxide and evaluation of drug effects on peroxide metabolism.
Key Features
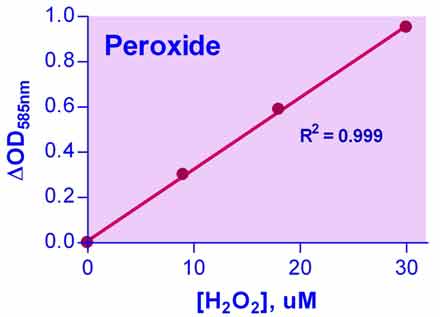
- Sensitive and accurate. Enhanced color intensity using sorbitol. Detection range 0.2 µM (7 ng/mL) to 30 µM (1,020 ng/mL) H2O2 in 96-well plate assay.
- Simple and high-throughput. The procedure involves addition of a single detection reagent and incubation for 30 min. Can be readily automated as a high-throughput assay in 96-well plates for thousands of samples per day.
Method
- OD585nm
Samples
- Serum, citrate-plasma, urine, cell lysate, culture media etc
Species
- All
Procedure
- 30 min
Size
- 250 tests
Detection Limit
- 0.2 µM
Shelf Life
- 12 months
More Details
Peroxide (e.g. hydrogen peroxide H2O2) is one of the key reactive oxygen species formed under oxidative stress conditions. High levels of peroxide formation have been linked to pathological conditions such as ageing, asthma, diabetes, atherosclerosis, cataract, inflammatory arthritis and neurodegenerative diseases. Simple, direct and automation-ready procedures for quantitative determination of peroxide find wide applications in research and drug discovery. BioAssay Systems peroxide assay kit is designed to measure peroxide concentration in biological samples without any pretreatment. The improved method utilizes the chromogenic Fe3+-xylenol orange reaction, in which a purple complex is formed when Fe2+ provided in the reagent is oxidized to Fe3+ by peroxides present in the sample. The intensity of the color, measured at 540-610nm, is an accurate measure of the peroxide level in the sample. The optimized formulation substantially reduces interference by substances in the raw samples.How can I measure peroxides in cell cultures?
1. Collect cells by centrifugation 1,000 g for 10 min at 4°C. For adherent cells, use a rubber policeman to harvest cells.
Homogenize or sonicate cell pellet in 1-2 mL of cold buffer containing 0.9% sodium chloride and 5 mM potassium phosphate, pH 7.4.
3. Centrifuge at 10,000 x g for 10 min at 4°C.
4. Use the clear supernatant for assay. If not assaying on the same day, freeze the sample at -80°C and use it within one month.
We want to determine hydrogen peroxide in a sample that likely contains other ingredients such as surfactant, protein, propylene glycol, phosphonic acid, Tween 20, all at 1% or lower. Hydrogen peroxide is probably present at around 1%. Is this assay kit suitable?
Yes, you need to dilute your sample 1,000 to 10,000-fold in water, so that the hydrogen peroxide concentration falls within the detection range of the assay (between 10 and 100 μM). This dilution would eliminate potential interference by other ingredients such as Tween-20.
I have archived some supernatant samples from macrophages that were stimulated for 24hrs with various different stimulants. Am I right in assuming that this kit has no requirement for cells to be functional and that levels of peroxide may loiter in supernatant samples for long enough so that they can be readily measured at 24hrs?
The peroxide assay kit does not require functional cells and peroxide levels can be measured in culture media. We do not know how stable peroxide is in cell culture media and this will likely depend on the cell type and culture conditions. In general, peroxide in serum and urine samples can be detected for several days, even after freezing, but levels may change over time.
My Samples contain sodium pyruvate, is there any way I could use them with this assay?
Sodium Pyruvate below concentrations of 1 mM does not interfere with the assay, if your samples fall in this range you may simply run them. If the sodium pyruvate concentration is between 1 mM and 5 mM, you will need to run the assay with a standard curve made in the media or buffer that contains the sodium pyruvate. The assay will not have as great of detection sensitivity, but will still function. If the sodium pyruvate concentration is greater than 5 mM, the samples will not work. You will need to dilute the samples for them to work.
Adhikari, B., et al (2020). Cold plasma seed priming modulates growth, redox homeostasis and stress response by inducing reactive species in tomato (Solanum lycopersicum). Free Radical Biology & Medicine, 156, 57-69. Assay: Peroxide in tomato leaves.
Liu, Z.,et al (2021). ROS-responsive and multifunctional anti-Alzheimer prodrugs: Tacrine-ibuprofen hybrids via a phenyl boronate linker. European Journal of Medicinal Chemistry, 212, 112997. Assay: Peroxide in antioxidant solution.
Zhang, Q., Zhang, F., Li, S., Liu, R., Jin, T., Dou, Y. & Zhang, J. (2019). A Multifunctional Nanotherapy for Targeted Treatment of Colon Cancer by Simultaneously Regulating Tumor Microenvironment. THERANOSTICS, 9(13), 3732-3753. Assay: Peroxide in Z-VAD-FMK cells.
Riedemann, I (2017). Insights Into the Mechanisms of Remote ischemic Preconditioning. PhD Dissertation the Kiel University. Assay: Peroxide in human endothelial cells.
Fedeles, B.I., et al. (2011). Chemical genetics analysis of an aniline mustard anticancer agent reveals complex I of the electron transport chain as a target. J Biol Chem 286(39):33910-20. Assay: Peroxide in human cancer cell.
Peng, A., et al. (2011). The green tea polyphenol (-)-epigallocatechin-3-gallate ameliorates experimental immune-mediated glomerulonephritis. Kidney Int. 80(6):601-11. Assay: Peroxide in mouse kidney tissue, urine, serum.
Fan, X., et al. (2010). H2O2-induced mitochondrial fragmentation in C2C12 myocytes. Free Radic Biol Med 49(11):1646-54. Assay: Peroxide in mouse cell culture supernatant.
Jawed, H., et al. (2010). N-(2-hydroxy phenyl) acetamide inhibits inflammation-related cytokines and ROS in adjuvant-induced arthritic (AIA) rats. Int Immunopharmacol 10(8):900-5. Assay: Peroxide in rat plasma/serum.
Toschi, A., et al. (2010). Phospholipase D-mTOR requirement for the Warburg effect in human cancer cells. Cancer Lett 299(1):72-9. Assay: Peroxide in human cells.
Cunningham, R.L., et al. (2009). Androgens induce dopaminergic neurotoxicity via caspase-3-dependent activation of protein kinase Cdelta. Endocrinology 150(12):5539-48. Assay: Peroxide in rat dopaminergic cell line n27.
Deshmane, S.L., et al. (2009). Activation of the oxidative stress pathway by HIV-1 Vpr leads to induction of hypoxia-inducible factor 1alpha expression. J Biol Chem 284(17):11364-73. Assay: Peroxide in human microglia cell line.
Minamishima, S., et al. (2009). Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation 120(10):888-96. Assay: Peroxide in mouse serum.
Nguyen GN et al (2009) Drought-Induced Oxidative Conditions in Rice Anthers Leading to a Programmed Cell Death and Pollen Abortion. Journal of Agronomy and Crop Science 195(3):157-164. Assay: Peroxide in plant rice anther.
Schnabl, K.L., et al. (2009). Gangliosides protect bowel in an infant model of necrotizing enterocolitis by suppressing proinflammatory signals. J Pediatr Gastroenterol Nutr 49(4):382-92. Assay: Peroxide in human intestinal tissue.
Shin, D., et al. (2009). Dicer ablation in oligodendrocytes provokes neuronal impairment in mice. Ann Neurol 66(6):843-57. Assay: Peroxide in mouse brain tissue.
Zhang, H., et al. (2009). Resveratrol improves endothelial function: role of TNF{alpha} and vascular oxidative stress. Arterioscler Thromb Vasc Biol 29(8):1164-71. Assay: Peroxide in mouse serum.
Lens, M., et al. (2008). Antioxidative capacity of C(60) (buckminsterfullerene) and newly synthesized fulleropyrrolidine derivatives encapsulated in liposomes. Biotechnol Appl Biochem 51(Pt 3):135-40. Assay: Peroxide in liposomes.
Sato, T., et al. (2008). Suppressive oligodeoxynucleotides inhibit silica-induced pulmonary inflammation. J Immunol 180(11):7648-54. Assay: Peroxide in mouse macrophages and culture supernatant.
Yu, C.L., et al. (2008). A novel caffeine dehydrogenase in Pseudomonas sp. strain CBB1 oxidizes caffeine to trimethyluric acid. J Bacteriol 190(2):772-6. Assay: Peroxide in Pseudomonas sp. purified enzyme.
Zhang, R., et al. (2008). In vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis 13(12):1465-78. Assay: Peroxide in human pancreatic cancer cells.
Hashimoto, T., et al. (2007). Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J 21(10):2602-12. Assay: Peroxide in rat L6 cell lysate.
To find more recent publications, please click here.
If you or your labs do not have the equipment or scientists necessary to run this assay, BioAssay Systems can perform the service for you.
– Fast turnaround
– Quality data
– Low cost
Please email or call 1-510-782-9988 x 2 to discuss your projects.
$409.00
For bulk quote or custom reagents, please email or call 1-510-782-9988 x 1.
Orders are shipped the same day if placed by 2pm PST
Shipping: RT
Carrier: Fedex
Delivery: 1-2 days (US), 3-6 days (Intl)
Storage: 4°C upon receipt
Related Products
You may also like…
| Name | SKU | Price | Buy |
|---|---|---|---|
| QuantiChrom™ Antioxidant Assay Kit | DTAC-100 | $379.00 | |
| QuantiChrom™ TBARS Assay Kit | DTBA-100 | $279.00 | |
| QuantiChrom™ Nitric Oxide Assay Kit | D2NO-100 | $379.00 | |
| QuantiChrom™ Peroxidase Assay Kit | D2PD-100 | $389.00 |
Why BioAssay Systems
Quality and User-friendly • Expert Technical Support • Competitive Prices • Expansive Catalogue • Trusted Globally
