EnzyChrom™ Triglyceride Assay Kit
Application
- For quantitative determination of triglyceride and evaluation of drug effects on triglyceride metabolism.
Key Features
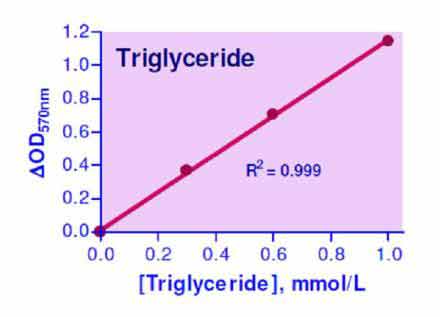
- Sensitive and accurate. Use as little as 10 µL samples. Linear detection range from 0.01 to 1.0 mmol/L (0.88 to 88.5 mg/dL) triglyceride.
- Simple and convenient. The procedure involves addition of a single working reagent and incubation for 30 min at room temperature, compatible for HTS assays.
- Improved reagent stability. The optimized formulation has greatly enhanced the reagent and signal stability.
Method
- OD570nm
Samples
- Serum, plasma etc
Species
- All
Procedure
- 30 min
Size
- 200 tests
Detection Limit
- 0.01 mmol/L (0.88 mg/dL)
Shelf Life
- 12 months
More Details
Triglyceride, also known as Triacyltriglyceride or Triacyl-glyceride, is the main constituent in vegetable oil and animal fats. Triglycerides play an important role as energy sources and transporters of dietary fat. In the human body, high levels of triglycerides in the bloodstream have been linked to atherosclerosis, heart disease, and pancreatitis. Simple, direct and automation-ready procedures for measuring triglyceride concentrations find wide applications in research and drug discovery. BioAssay Systems triglyceride assay uses a single Working Reagent that combines triglyceride hydrolysis and glycerol determination in one step, in which a dye reagent is oxidized to form a colored product. The color intensity at 570nm is directly proportional to triglyceride concentration in the sample.How to extract triglycerides from tissue?
1. Prepare extraction buffer with 5 vol isopropanol, 2 vol water, 2 vol Triton X-100.
In a 1.5 mL Eppendorf tube, add 50 µL/mg tissue of the extraction buffer. Vortex for at least 30 sec.
3. Centrifuge 5 min at 14,000 rpm on a table centrifuge.
4. Remove supernatant, dilute 5× with dH2O and perform triglyceride assay using the ETGA-100 Kit.
I want to quantify triglycerides in algae and yeasts. The kit’s datasheet explains to use Triton X-100 to solubilize cells and solid components. Will this also work for Algae and Yeast?
Since yeast and algae have cell walls, Triton X-100 may not be sufficient to solubilize these cells. Sigma Aldrich offers a couple reagents for lysis of yeast and plant cells which should work, although we have not tested if these reagents would interfere with the assay.
Alternatively,you could try using sonication/ mechanical homogenization to disrupt the cells.
Jiang, S. et al (2021). Associations of Circulating Irisin with FNDC5 Expression in Fat and Muscle in Type 1 and Type 2 Diabetic Mice. Biomolecules, 11(2), 322. Assay: tryglycerides in mouse plasma.
Lee, J. et al (2020). Pharmaceutical Efficacy of Gypenoside LXXV on Non-Alcoholic Steatohepatitis (NASH). Biomolecules, 10(10), E1426. Assay: tryglycerides in mouse liver tissue.
Andres-Hernando, A. et al (2020). Deletion of Fructokinase in the Liver or in the Intestine Reveals Differential Effects on Sugar-Induced Metabolic Dysfunction. Cell metabolism, 32(1), 117-127. Assay: tryglycerides in mouse liver tissue.
Jhun, J. et al (2021). GRIM19 Impedes Obesity by Regulating Inflammatory White Fat Browning and Promoting Th17/Treg Balance. Cells, 10(1), E162. Assay: tryglycerides in mouse liver tissue.
Moorhead, S. G.et al. Variation of body condition and plasma energy substrates with life stage, sex, and season in wild-sampled nurse sharks Ginglymostoma cirratum. Journal of fish biology. Assay: tryglycerides in nurse shark plasma.
Andres-Hernando, A. et al. Vasopressin mediates fructose-induced metabolic syndrome by activating the V1b receptor. JCI insight, 140848. Assay: tryglycerides in mouse liver tissue.
Chi, Y. et al (2020). PHTF2 Regulates Lipids Metabolism in Gastric Cancer. Aging, 12(8), 6600-6610. Assay: tryglycerides in human gastric cells.
Shin, M. K., Yang, S. M., & Han, I. S. (2020). Capsaicin suppresses liver fat accumulation in high-fat diet-induced NAFLD mice. Animal cells and systems, 24(4), 214-219. Assay: tryglycerides in mouse plasma.
Ghazali, R. et al (2020). High omega arachidonic acid/docosahexaenoic acid ratio induces mitochondrial dysfunction and altered lipid metabolism in human hepatoma cells. World journal of hepatology, 12(3), 84. Assay: tryglycerides in human hepatoma.
Shi, Y., & Leung, S. W. S. Long-term Nitric Oxide Synthase Inhibition Prevents 17β-estradiol-induced Suppression of Cyclooxygenase-Dependent Contractions and Enhancement of Endothelium-Dependent Hyperpolarization-Like Relaxation in Mesenteric Arteries of Ovariectomized Rats. European journal of pharmacology, 882, 173275. Assay: tryglycerides in rat plasma.
Han, Y. H., Kim, H. J., & Lee, M. O. RORα regulates hepatic lipolysis by inducing transcriptional expression of PNPLA3 in mice. Molecular and cellular endocrinology, 111122. Assay: tryglycerides in mouse hepatocytes.
Hirsova, P. et al (2020). Hepatocyte Apoptosis Is Tumor Promoting in Murine Nonalcoholic Steatohepatitis. Cell death & disease, 11(2), 80. Assay: tryglycerides in mouse liver homogenate.
Nguyen, H. T. L., Kasapis, S., & Mantri, N. (2021). Physicochemical Properties and Effects of Honeys on Key Biomarkers of Oxidative Stress and Cholesterol Homeostasis in HepG2 Cells. Nutrients, 13(1), 151. Assay: tryglycerides in human liver cells.
Bergmans, R. S. et al (2020). Comparison of Cricket Diet With Peanut-Based and Milk-Based Diets in the Recovery From Protein Malnutrition in Mice and the Impact on Growth, Metabolism and Immune Function. PloS one, 15(6), e0234559. Assay: tryglycerides in mouse serum.
Seo, B. et al (2020). Roseburia spp. Abundance Associates with Alcohol Consumption in Humans and Its Administration Ameliorates Alcoholic Fatty Liver in Mice. Cell host & microbe, 27(1), 25. Assay: tryglycerides in mouse liver.
Lambert, K. et al (2020). Biocompatible Modified Water as a Non-Pharmaceutical Approach to Prevent Metabolic Syndrome Features in Obesogenic Diet-Fed Mice. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association, 141, 111403. Assay: tryglycerides in mouse liver.
Ju, U. I. et al (2020). Neddylation of Sterol Regulatory Element-Binding Protein 1c Is a Potential Therapeutic Target for Nonalcoholic Fatty Liver Treatment. Cell death & disease, 11(4), 283. Assay: tryglycerides in human serum and liver.
Colijn, S. et al (2020). Cell-specific and Athero-Protective Roles for RIPK3 in a Murine Model of Atherosclerosis. Disease models & mechanisms, 13(1), dmm041962. Assay: tryglycerides in mouse plasma.
Azar, S. et al. “Reversal of diet-induced hepatic steatosis by peripheral CB1 receptor blockade in mice is p53/miRNA-22/SIRT1/PPARα dependent.” Molecular metabolism 42: 101087. Assay: tryglycerides in mouse liver.
Pereira, R. O. et al (2021). OPA1 Deletion in Brown Adipose Tissue Improves Thermoregulation and Systemic Metabolism via FGF21. bioRxiv, 2021-01. Assay: tryglycerides in mouse liver and serum.
Higashi, K., Mori, A., Sakamoto, K., Ishii, K., & Nakahara, T. (2019). Probucol Slows the Progression of Cataracts in Streptozotocin-Induced Hyperglycemic rats. Pharmacology, 103(3-4), 212-219. Assay: Triglyceride in rat plasma.
Zagury, Y., Chen, S., Edelman, R., Karnieli, E., & Livney, Y. D. (2019). beta-Lactoglobulin delivery system for enhancing EGCG biological efficacy in HFD obesity mice model. Journal of Functional Foods, 59, 362-370. Assay: Triglyceride in mouse liver.
Zhang, X., Wang, S., Hu, L., Wang, J., Liu, Y., & Shi, P. (2019). Gemfibrozil reduces lipid accumulation in SMMC-7721 cells via the involvement of PPARalpha and SREBP1. Experimental and therapeutic medicine, 17(2), 1282-1289. Assay: Triglyceride in human hepatoma SMMC-7721 cells.
Zuo, L., Ge, S., Ge, Y., Li, J., Zhu, B., Zhang, Z., & Li, S. (2019). The adipokine metrnl ameliorates chronic colitis in Il-10-/-mice by attenuating mesenteric adipose tissue lesions during spontaneous colitis. Journal of Crohn’s and Colitis. Assay: Triglyceride in mouse & human mesenteric adipose tissue.
Ding, Y., Cui, J., Wang, Q., Shen, S., Xu, T., Tang, H., & Wu, X. (2018). The Vitamin K Epoxide Reductase Vkorc1l1 Promotes Preadipocyte Differentiation in Mice. Obesity, 26(8), 1303-1311. Assay: Triglyceride in mouse adipocytes.
Feng, J., Li, L., Ou, Z., Li, Q., Gong, B., Zhao, Z., & Yang, X. (2018). IL-25 stimulates M2 macrophage polarization and thereby promotes mitochondrial respiratory capacity and lipolysis in adipose tissues against obesity. Cellular & molecular immunology, 15(5), 493. Assay: Triglyceride in mouse liver and serum.
Lambert, K., Hokayem, M., Thomas, C., Fabre, O., Cassan, C., Bourret, A., & Avignon, A. (2018). Combination of nutritional polyphenols supplementation with exercise training counteracts insulin resistance and improves endurance in high-fat diet-induced obese rats. Scientific reports, 8(1), 2885. Assay: Triglyceride in rat liver homogenate.
Buss, L. A., & Dachs, G. U. (2017). Voluntary exercise slows breast tumor establishment and reduces tumor hypoxia in ApoE-/- mice. Journal of Applied Physiology, 124(4), 938-949. Assay: Triglyceride in mouse serum.
Gallagher, A. J., Skubel, R. A., Pethybridge, H. R., & Hammerschlag, N. (2017). Energy metabolism in mobile, wild-sampled sharks inferred by plasma lipids. Conservation Physiology, 5(1). Assay: Triglyceride in shark plasma.
Kim, S. H., Kim, G., Han, D. H., Lee, M., Kim, I., Kim, B., & Bae, S. H. (2017). Ezetimibe ameliorates steatohepatitis via AMP activated protein kinase-TFEB-mediated activation of autophagy and NLRP3 inflammasome inhibition. Autophagy, 13(10), 1767-1781. Assay: Triglyceride in mouse Liver tissue.
Lee, H., Shim, E. H., Lee, M. S., & Myung, C. S. (2017). Traditional medicine, Sobokchukeo-Tang, modulates the inflammatory response in adipocytes and macrophages. Molecular medicine reports, 15(1), 117-124. Assay: Triglyceride in RAW264.7 and THP-1 cells cell lysate.
Sadowska, J., Gebczynski, A. K., & Konarzewski, M. (2017). Metabolic risk factors in mice divergently selected for BMR fed high fat and high carb diets. PloS one, 12(2), e0172892. Assay: Triglyceride in mouse serum.
Wang, R. Y., Abbott, R. D., Zieba, A., Borowsky, F. E., & Kaplan, D. L. (2017). Development of a three-dimensional adipose tissue model for studying embryonic exposures to obesogenic chemicals. Annals of biomedical engineering, 45(7), 1807-1818. Assay: Triglyceride in human embryonic derived stem cells.
Choi, Y., Abdelmegeed, M. A., & Song, B. J. (2016). Preventive effects of dietary walnuts on high-fat-induced hepatic fat accumulation, oxidative stress and apoptosis in mice. The Journal of nutritional biochemistry, 38, 70-80. Assay: Triglyceride in mouse liver tissue.
Hernandez-Vazquez, A. D. J., Garcia-Sanchez, J. A., Moreno-Arriola, E., Salvador-Adriano, A., Ortega-Cuellar, D., & Velazquez-Arellano, A. (2016). Thiamine deprivation produces a liver ATP deficit and metabolic and genomic effects in mice: findings are parallel to those of biotin deficiency and have implications for energy disorders. Lifestyle Genomics, 9(5-6), 287-299. Assay: Triglyceride in mouse liver tissue.
Liss, S. A., Lamer, J. T., Sass, G. G., & Suski, C. D. (2016). Physiological consequences of hybridization: early generation backcrossing decreases performance in invasive bigheaded carps. Journal of Freshwater Ecology, 31(4), 543-554. Assay: Triglyceride in big-headed and silver carp blood.
Marques, T. M., Patterson, E., Wall, R., O’Sullivan, O., Fitzgerald, G. F., Cotter, P. D., & Stanton, C. (2016). Influence of GABA and GABA-producing Lactobacillus brevis DPC 6108 on the development of diabetes in a streptozotocin rat model. Beneficial microbes, 7(3), 409-420. Assay: Triglyceride in mouse.
Patterson, T. G., Freeman, T., & Flook, J. A. (2016). Colorimetric determination of the total oil content of a plant tissue sample using alkaline saponification. U.S. Patent No. 9,395,377. Assay: Triglyceride in human kernel.
Zawieja, S. D., Wang, W., Chakraborty, S., Zawieja, D. C., & Muthuchamy, M. (2016). Macrophage alterations within the mesenteric lymphatic tissue are associated with impairment of lymphatic pump in metabolic syndrome. Microcirculation, 23(7), 558-570. Assay: Triglyceride in mouse serum.
Cylwik B et al (2012) Relationship between CDT and disease activity in rheumatoid arthritis. Z Rheumatol 71(3):220-3. Assay: Triglyceride in rat blood.
Bytautiene, E., et al. (2011). Prepregnancy obesity and sFlt1-induced preeclampsia in mice: developmental programming model of metabolic syndrome. Am J Obstet Gynecol 204(5):398 e1-8. Assay: Triglyceride in mouse serum.
Guo H, et al (2011). Anthocyanin inhibits high glucose-induced hepatic mtGPAT1 activation and prevents fatty acid synthesis through PKCzeta. J Lipid Res. 52(5):908-22. Assay: Triglyceride in mouse, human cellular fractions.
Orban T, et al (2011). Retinyl ester storage particles (retinosomes) from the retinal pigmented epithelium resemble lipid droplets in other tissues. J Biol Chem. 286(19):17248-58. Assay: Triglyceride in cow retinal pigment epithelium.
Uddin MJ, et al (2011). Detection of quantitative trait loci affecting serum cholesterol, LDL, HDL, and triglyceride in pigs. BMC Genet.12:62. Assay: Triglyceride in pig serum.
Hu, M. et al. (2010). Effect of prolonged starvation on body weight and blood-chemistry in two horseshoe crab species: Tachypleus tridentatus and Carcinoscorpius rotundicauda (Chelicerata: Xiphosura). J. Exp. Marine Biol. Ecology 395(1-2):112-119. Assay: Triglyceride in horseshoe crab plasma.
Kim HS et al (2010). Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab. 12(3):224-36. Assay: Triglyceride in human HepG2 hepatocellular carcinoma cells.
Oh TW, et al (2010). Semipurified fractions from the submerged-culture broth of Agaricus blazei Murill reduce blood glucose levels in streptozotocin-induced diabetic rats. J Agric Food Chem. 58(7):4113-9. Assay: Triglyceride in rat plasma.
Tam J et al (2010). Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 120(8):2953-66. Assay: Triglyceride in mouse primary hepatocytes.
Tucci, S et al (2010). Medium-chain triglycerides impair lipid metabolism and induce hepatic steatosis in very long-chain acyl-CoA dehydrogenase (VLCAD)-deficient mice. Mol Genet Metab 101(1): 40-47 . Assay: Triglyceride in mouse Liver.
Lee, SM et al (2008).GCG-rich tea catechins are effective in lowering cholesterol and triglyceride concentrations in hyperlipidemic rats. Lipids 43(5): 419-429. . Assay: Triglyceride in rat plasma.
To find more recent publications, please click here.
If you or your labs do not have the equipment or scientists necessary to run this assay, BioAssay Systems can perform the service for you.
– Fast turnaround
– Quality data
– Low cost
Please email or call 1-510-782-9988 x 2 to discuss your projects.
$499.00
For bulk quote or custom reagents, please email or call 1-510-782-9988 x 1.
Orders are shipped the same day if placed by 2pm PST
Shipping: On Ice
Carrier: Fedex
Delivery: 1-2 days (US), 3-6 days (Intl)
Storage: -20°C upon receipt
Related Products
You may also like…
| Name | SKU | Price | Buy |
|---|---|---|---|
| EnzyChrom™ Glycerol Assay Kit | EGLY-200 |
$489.00 |
Why BioAssay Systems
Quality and User-friendly • Expert Technical Support • Competitive Prices • Expansive Catalogue • Trusted Globally
