Filter Plate Solubility Service
Summary
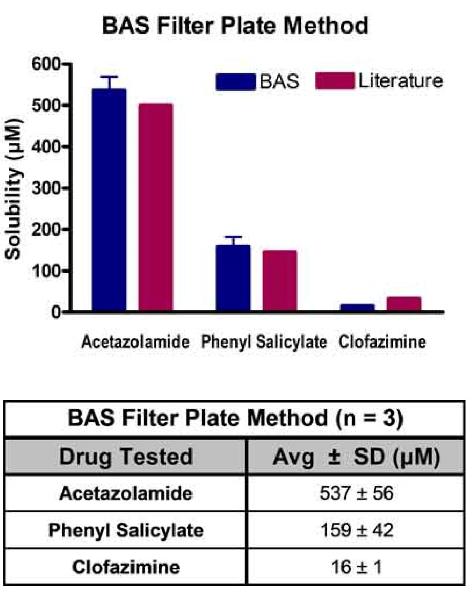
The solubility of a drug compound plays a significant role in absorption and is an important factor to consider during development. Quantitative measurement of compound solubility is an ideal early test to perform when screening for potential drug candidates.
BioAssay Systems (BAS) offers two methods for quantifying the solubility of drug compounds: filter plate solubility testing and shake flask solubility testing. This information page details the Filter Plate Method.
BioAssay Systems (BAS) offers two methods for quantifying the solubility of drug compounds: filter plate solubility testing and shake flask solubility testing. This information page details the Filter Plate Method.
Method
In MultiScreen filter plate, the compound is mixed with a solvent of customer’s choice. The plate is gently shaken for 1.5 hours at ambient temperature (or desired temperature). The solution is vacuum filtered. Absorbance spectrum is run on the filtrate together with the test compound standards. Concentration of the filtrate is calculated using the slope of the standards.
Our Services
- As little as 50 to 1000 µg compound is needed.
- No structure information is required.
- If compound is in DMSO, 10mM or higher concentration is preferred.
- Report will be available within 48 hours.
Please email or call us at 1-510-782-9988 x 2 to discuss your service needs.