EnzyLight™ ATP Assay Kit
Application
- For rapid, quantitative, bioluminescent determination of ATP and evaluation of drug effects on ATP metabolism.
Key Features
- Safe. Non-radioactive assay.
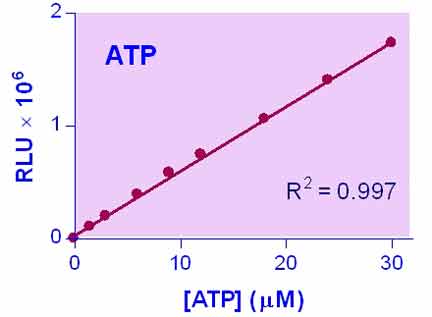
- Sensitive and accurate. As low as 0.02 µM ATP or a single cell can be quantified.
- Homogeneous and convenient. “Mix-incubate-measure” type assay. No wash and reagent transfer steps are involved.
- Robust and amenable to HTS: Z factors of > 0.5 are routinely observed in 96-well and 384-well plates. Can be readily automated on HTS liquid handling systems.
Method
- Luminescence
Samples
- Cells etc
Species
- All
Procedure
- 10 min
Size
- 100 tests
Detection Limit
- 0.02 µM
Shelf Life
- 12 months
More Details
Adenosine 5’-triphosphate (ATP) is the chemical energy for cellular metabolism and is often referred to as “energy currency” of the cell. ATP is produced only in living cells during photosynthesis and cellular respiration and consumed in cellular processes including biosynthetic reactions, motility and cell division. It is a key indicator of cellular activity and has been utilized as a measure of cell viability and cytotoxicity in research and drug discovery. BioAssay Systems’ EnzyLight™ ATP Assay Kit provides a rapid method to measure intracellular ATP. The single working reagent lyses cells to release ATP, which, in the presence of luciferase, immediately reacts with the Substrate D-luciferin to produce light. The light intensity is a direct measure of intracellular ATP concentration. This non-radioactive, homogeneous cell-based assay is performed in microplates. The reagent is compatible with all liquid handling systems for high-throughput screening applications in 96-well and 384-well plates.Can this kit be used to adhesive cells?
This kit is suitable for adherent cells. The assay buffer contains 0.5 % Triton X-100 that will efficiently lyse the cells.
Is the lysis buffer in the kit suitable for Bradford protein assay?
The final Triton X-100 concentration in the lysate will be around 0.25 % which would interfere with the Bradford assay. Therefore, I would recommend diluting the lysate further. The commonly used BCA assay is not compatible, because of the high amount of mercaptoethanol in the assay buffer (70 mM).
Maeda, Y. et al (2020). Anti-cancer strategy targeting the energy metabolism of tumor cells surviving a low-nutrient acidic microenvironment. Molecular Metabolism, 42. Assay: ATP in human lung cancer cell.
Jackisch, L. et al (2020). Tunicamycin-Induced Endoplasmic Reticulum Stress Mediates Mitochondrial Dysfunction in Human Adipocytes. The Journal of clinical endocrinology and metabolism, 105(9), dgaa258. Assay: ATP in human adipocytes.
Wang, Y. et al (2020). IF1 connects obesity and insulin resistance through mitochondrial reprogramming in association with ANT2. Assay: ATP in mouse muscle tissue.
Coyotl, E. et al (2020). Antimicrobial Peptide against Mycobacterium Tuberculosis That Activates Autophagy Is an Effective Treatment for Tuberculosis. Pharmaceutics, 12(11). Assay: ATP in human HEK293T cells.
Zhang, Z. et al (2020). Bcl-2 Proteins Regulate Mitophagy in Lipopolysaccharide-Induced Acute Lung Injury via PINK1/Parkin Signaling Pathway. Oxidative Medicine and Cellular Longevity, 2020. Assay: ATP in human A549 Cell.
Le, Jiamei, et al (2019). Regulation of microbiota-GLP1 axis by Sennoside A (SA) in diet-induced obese mice. Acta Pharmaceutica Sinica B. Assay: ATP in mice tissues.
Cai, J-G., et al (2018). Cytotoxicity of Malondialdehyde and Cytoprotective Effects of Taurine via Oxidative Stress and PGC-1alpha Signal Pathway in C2C12 Cells. Molecular Biology 52.4: 532-542. Assay: ATP in mouse cells.
Fan, Fengyan, et al (2018). Effects of red blood cell supernatants on hypoxia/reoxygenation injury in H9C2 cells. Intl.J. Clin. Exp. Med.11.4: 3612-3619. Assay: ATP in rat H9C2 cells.
Hashim, Mahira, et al (2018). Inhibition of SNAT5 induces incretin-responsive state from incretin-unresponsive state in pancreatic beta-cells: study of beta-cell spheroid clusters as a model. Diabetes 67.9: 1795-1806. Assay: ATP in mice pancreatic beta-cells.
Hoque, SA Masudul, et al (2018). Mitochondrial protein turnover is critical for granulosa cell proliferation and differentiation in antral follicles. Journal of the Endocrine Society 3.2: 324-339. Assay: ATP in mouse granulosa cell.
Ouyang, Pengfei, et al (2018). Increasing oxygen availability for improving poly (3-hydroxybutyrate) production by Halomonas. Metabolic engineering 45: 20-31. Assay: ATP in Halomonas bluephagenesis cells.
Pan, Jin-Xiu, et al (2018). APP promotes osteoblast survival and bone formation by regulating mitochondrial function and preventing oxidative stress. Cell death & disease 9.11: 1077. Assay: ATP in mice cells.
Shen, W., et al (2018). Mitochondria-mediated disturbance of fatty acid metabolism in proximal tubule epithelial cells leads to renal interstitial fibrosis. Eur Rev Med Pharmacol Sci 22.3: 810-819. Assay: ATP in mice serum.
Sun, Yongning, et al (2018). Restoration of GLP-1 secretion by Berberine is associated with protection of colon enterocytes from mitochondrial overheating in diet-induced obese mice. Nutrition & diabetes 8.1: 53. Assay: ATP in mice tissues.
Zhong, Hanhui, et al (2018). Propofol inhibits parthanatos via ROS-ER-calcium-mitochondria signal pathway in vivo and vitro. Cell death & disease 9.10: 932. Assay: ATP in mice cells.
Lin, Zou, et al (2017). Protective effect of alpha-lipoic acid against antimycin A cytotoxicity in MC3T3-E1 osteoblastic cells. Cell Stress and Chaperones 22.1: 5-13. Assay: ATP in mouse cells.
Rink, Cameron, et al (2017). Glutamate oxaloacetate transaminase enables anaplerotic refilling of TCA cycle intermediates in stroke-affected brain. The FASEB Journal 31.4: 1709-1718. Assay: ATP in mice brain tissues.
Aldonza, Mark Borris D., et al (2016). Multiplicity of acquired cross-resistance in paclitaxel-resistant cancer cells is associated with feedback control of TUBB3 via FOXO3a-mediated ABCB1 regulation. Oncotarget 7.23: 34395. Assay: ATP in human cells.
Joshi, A., et al (2016). Nuclear ULK1 promotes cell death in response to oxidative stress through PARP1. Cell death and differentiation 23.2: 216. Assay: ATP in murine cells.
Suh, Kwang Sik, Suk Chon, and Eun Mi Choi (2016). Luteolin alleviates methylglyoxal-induced cytotoxicity in osteoblastic MC3T3-E1 cells. Cytotechnology 68.6: 2539-2552. Assay: ATP in mouse cells.
Aflaki E, et al (2011). Triacylglycerol accumulation activates the mitochondrial apoptosis pathway in macrophages. J Biol Chem. 286(9):7418-28. Assay: ATP in mouse macrophage.
Choi EM, Lee YS (2011). Protective effect of apocynin on antimycin A-induced cell damage in osteoblastic MC3T3-E1 cells. J Appl Toxicol. 32(9):714-21. Assay: ATP in mouse cell.
Khuda-Buksh, AR. et al. (2011). Analysis of the capability fo ultra-highly diluted glucose to increase glucose uptake in arsenite-streesed bacteria Escherichia coli. J, Chin. Integrative Medicine 9(8): 901-912. Assay: ATP in bacteria cell lysate.
Khuda-Buksh, AR. et al. (2011). Analysis of the capability fo ultra-highly diluted glucose to increase glucose uptake in arsenite-streesed bacteria Escherichia coli. J, Chin. Integrative Medicine 9(8): 901-912. Assay: ATP in bacteria.
Methods for treatment of oncological disorders using an epimetabolic shifter (coenzyme q10). US Patent Appl. 20110027247. Assay: ATP in mouse cell.
Narain, NR, McCook, JP. (2011). Methods for treatment of oncological disorders using an epimetabolic shifter (coenzyme q10). US Patent Appl. 20110027247. Assay: ATP in human cell.
Ponnusamy M, et al (2011). P2X7 receptors mediate deleterious renal epithelial-fibroblast cross talk. Am J Physiol Renal Physiol. 300(1):F62-70. Assay: ATP in rat fibroblast.
Belleannee C, et al (2010). Role of purinergic signaling pathways in V-ATPase recruitment to apical membrane of acidifying epididymal clear cells. Am J Physiol Cell Physiol. 298(4):C817-30. Assay: ATP in rat tube.
Chandak PG, et al (2010). Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase. J Biol Chem.285(26):20192-201. Assay: ATP in mouse cells.
Dvoriantchikova G, et al (2010). Liposome-delivered ATP effectively protects the retina against ischemia-reperfusion injury. Mol Vis.16:2882-90. Assay: ATP in mouse retina.
Sugimoto S, et al (2009). Apyrase treatment prevents ischemia-reperfusion injury in rat lung isografts. J Thorac Cardiovasc Surg. 138(3):752-9. Assay: ATP in rat lung tissue.
Schwarzer C, et al (2008). Oxidative stress caused by pyocyanin impairs CFTR Cl(-) transport in human bronchial epithelial cells. Free Radic Biol Med. 45(12):1653-62. Assay: ATP in human cell.
To find more recent publications, please click here.
If you or your labs do not have the equipment or scientists necessary to run this assay, BioAssay Systems can perform the service for you.
– Fast turnaround
– Quality data
– Low cost
Please email or call 1-510-782-9988 x 2 to discuss your projects.
$419.00
For bulk quote or custom reagents, please email or call 1-510-782-9988 x 1.
Orders are shipped the same day if placed by 2pm PST
Shipping: On Ice
Carrier: Fedex
Delivery: 1-2 days (US), 3-6 days (Intl)
Storage: -20°C upon receipt
Related Products
You may also like…
| Name | SKU | Price | Buy |
|---|---|---|---|
| EnzyLight™ ADP Assay Kit | EADP-100 |
$419.00 |
|
| EnzyLight™ ATP Assay Kit | EATP-100 |
$419.00 |
|
| EnzyLight™ ADP/ATP Ratio Assay Kit | ELDT-100 |
$459.00 |
Why BioAssay Systems
Quality and User-friendly • Expert Technical Support • Competitive Prices • Expansive Catalogue • Trusted Globally
