Membrane Permeability Services
Summary
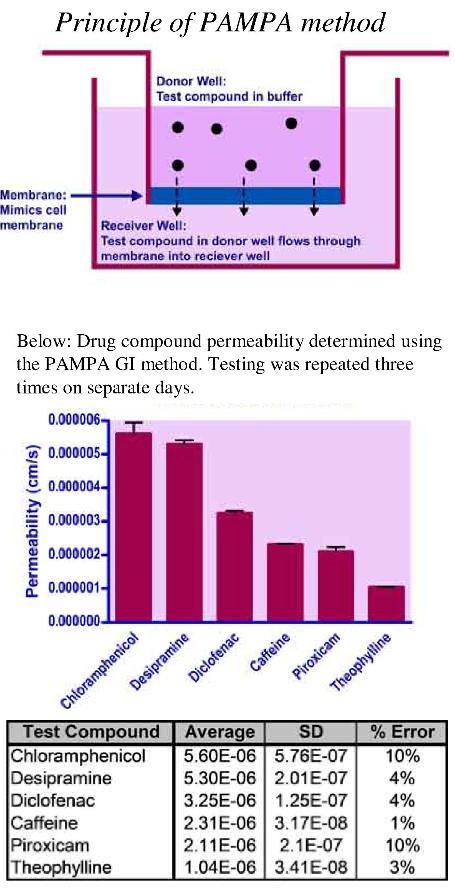
Method
A membrane mimicking solution and full permeability control solution are loaded on to separate well membranes of a PAMPA donor plate. The donor plate is then loaded with the desired concentration of test compound in a buffer of customer’s choice. The receiver plate is loaded with either the same buffer or another of the customer’s choice. The donor plate is gently placed into the receiver plate and incubated for a set duration at ambient temperature (or desired temperature).
After the incubation, the solutions in the receiver plate are collected and analyzed by UV-spectroscopy alongside test compound standards in DMSO. The test compound permeability is calculated by comparing the membrane mimicking system to that of the full permeability control.
Our Service
- As little as 50 to 1000 µg compound is needed.
- No structure information is required.
- If compound is in DMSO, 10 mM or higher concentration is preferred.
- Report will be available within 72 hours.