Aldehyde Dehydrogenase Inhibitor Screening Service
Summary
Before determining the IC50‘s for the Aldehyde Dehydrogenase (ALDH) inhibitor, we performed some initial experiments to establish appropriate assay conditions. We assessed conditions for baker’s yeast potassium activated ALDH. First, we titrated the enzyme using 20 mM acetaldehyde as the substrate. From these titrations, we decided to use 1.3 U/reaction of ALDH. We next titrated the solvent DMSO to determine any inhibitory effects it may have on ALDH. The Km of acetaldehyde for ALDH was then measured in the presence of the highest tolerable DMSO concentration. Finally, the IC50 for a known ALDH inhibitor, disulfiram, was measured. We determined the maximum DMSO concentration for the test compounds to be 100 v% (final reaction concentration of 5 v%). We determined the acetaldehyde Km to be 900 ± 92 µM. Using 900 µM acetaldehyde, we observed an IC50 = 2.65 µM for disulfiram.
Results
ALDH Titration
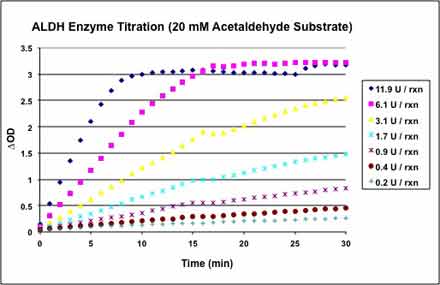
In order to determine an appropriate amount of ALDH to use for the IC50 determinations, we titrated the ALDH enzyme. ALDH was titrated from 12 U/rxn. The titration was performed in the presence of 5 v% DMSO using 20 mM final [acetaldehyde] as the substrate. The ALDH titration was performed in a clear 96-well plate. From the results, we opted to use 1.3 U/rxn ALDH since at this concentration the reaction would remain linear for at least 30 min.
In order to determine an appropriate amount of ALDH to use for the IC50 determinations, we titrated the ALDH enzyme. ALDH was titrated from 12 U/rxn. The titration was performed in the presence of 5 v% DMSO using 20 mM final [acetaldehyde] as the substrate. The ALDH titration was performed in a clear 96-well plate. From the results, we opted to use 1.3 U/rxn ALDH since at this concentration the reaction would remain linear for at least 30 min.
Km of of Acetaldehyde
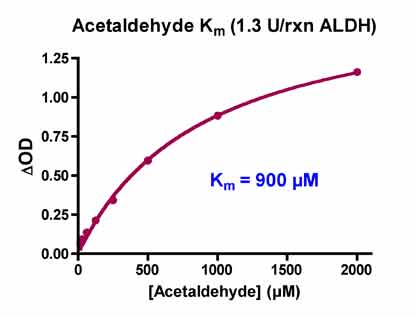
In order to determine the Km of acetaldehyde for ALDH, we titrated acetaldehyde from 0-2 mM with 1.3 U/rxn ALDH and computed the ΔOD (OD30min – OD0min), which is proportional to the rate of reaction, for each acetaldehyde concentration. The ΔOD’s were then plotted versus [acetaldehyde] and the Km was computed using Prism 4 (GraphPad Software Inc.). The ALDH Km determination was performed in the presence of 5 v% DMSO (final reaction concentration). We found the Km = 900 µM acetaldehyde.
In order to determine the Km of acetaldehyde for ALDH, we titrated acetaldehyde from 0-2 mM with 1.3 U/rxn ALDH and computed the ΔOD (OD30min – OD0min), which is proportional to the rate of reaction, for each acetaldehyde concentration. The ΔOD’s were then plotted versus [acetaldehyde] and the Km was computed using Prism 4 (GraphPad Software Inc.). The ALDH Km determination was performed in the presence of 5 v% DMSO (final reaction concentration). We found the Km = 900 µM acetaldehyde.
DMSO Titration
In order to determine tolerance for DMSO of ALDH, we ran DMSO titrations from 0-5 v% in the reaction. For these titrations we used 1.3U ALDH per reaction. The reaction was initiated by adding 900 µM final [acetaldehyde] and was allowed to proceed for 30 min. We then computed the ΔOD (OD30min – OD0min), which is proportional to the rate of reaction, for each DMSO concentration. The ΔOD was then plotted versus DMSO concentration. From the result we discovered that at substrate concentration of 900 µM acetaldehyde, DMSO did not affect the activity of ALDH. For the ALDH inhibitor, we decided to move forward with 100 v% DMSO as the inhibitor solvent, which would result in a final 5 v% DMSO per reaction.
In order to determine tolerance for DMSO of ALDH, we ran DMSO titrations from 0-5 v% in the reaction. For these titrations we used 1.3U ALDH per reaction. The reaction was initiated by adding 900 µM final [acetaldehyde] and was allowed to proceed for 30 min. We then computed the ΔOD (OD30min – OD0min), which is proportional to the rate of reaction, for each DMSO concentration. The ΔOD was then plotted versus DMSO concentration. From the result we discovered that at substrate concentration of 900 µM acetaldehyde, DMSO did not affect the activity of ALDH. For the ALDH inhibitor, we decided to move forward with 100 v% DMSO as the inhibitor solvent, which would result in a final 5 v% DMSO per reaction.

Disulfiram IC50 Determination
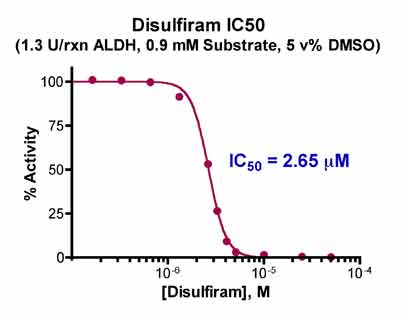
The IC50 determination required two steps: 1) 20 min pre-incubation of 45 µL ALDH (1.3 U/rxn) with 5 µL disulfiram in 100% DMSO, and 2) aldehyde dehydrogenase reaction with 900 µM final [acetaldehyde] for 30 min at 25°C. We titrated disulfiram from 0-50 µM and computed the ΔOD for each inhibitor concentration. The ΔOD’s were then plotted versus the inhibitor concentration and the IC50 was computed using Prism 4 (GraphPad Software Inc.). We observed an IC50 = 2.65 µM.
The IC50 determination required two steps: 1) 20 min pre-incubation of 45 µL ALDH (1.3 U/rxn) with 5 µL disulfiram in 100% DMSO, and 2) aldehyde dehydrogenase reaction with 900 µM final [acetaldehyde] for 30 min at 25°C. We titrated disulfiram from 0-50 µM and computed the ΔOD for each inhibitor concentration. The ΔOD’s were then plotted versus the inhibitor concentration and the IC50 was computed using Prism 4 (GraphPad Software Inc.). We observed an IC50 = 2.65 µM.
Please email or call 1-510-782-9988 x 2 to request assay service.
Customer Testimonials
"The guys at BioAssay Systems know their science! I had to develop a bioassay for our company and Robert was very quick to respond to keep us up to date. They are knowledgeable and very resourceful. They directed us to one of the bulk suppliers that could give us a really good deal on our active ingredient. They keep very detailed records for our research needs. I would recommend working with BioAssay Systems on your assay development projects!"

"It is a pleasure working together with BioAssay Systems. I brought samples to their Hayward location and received the results in less than 24 hr. The discussion with Robert Z was courteous, open, and with a depth of knowledge. Robert has a PhD from Stanford. Also, Frank Huang, the CEO, had sufficient scientific curiosity to try out our cleanser and provide feedback! This is beyond the call of customer relations. BioAssay Systems is a company with a lot of expertise and a friendly, responsive approach to problem solving. I will enthusiastically continue to work with them to carry out our research."

"I would like to show my appreciation to the Bioassay Systems service team. We are developing an assay to measure the change in lactate concentration of single cell samples using fluorescent microscopy. The BioAssay Systems team was quite helpful in assisting us to optimize their fluorescent L-Lactate assay kit for our unique experimental set-up. The BioAssay Systems team is always friendly and knowledgeable. We know that we can rely on them for fast and professional service."

"While developing biodegradable coatings for Drug Eluting Stents I worked with Bob to develop an accurate, precise and sensitive assay for determining the amount of polymer remaining in tissue. It was a pleasure to work with Bob and his team, they are very thorough, they understand the science very well and they always deliver on time and at the right price. I would highly recommend anyone using BioAssay Systems services in the future."

